The field of vascular biology is broad, as the vascular system plays a role in the development and progression of many diseases and pathologies. Vascular research aims to uncover the underlying mechanisms of vessels as they relate to organ-specific diseases.
Preclinical vascular research is important for a wide range of diseases but demands high resolution in small animal models. Most conventional ultrasound systems are limited to frequencies in the 2-15 MHz range and do not afford resolution needed for small animal preclinical research [1, 2].
For this reason, VisualSonics has developed ultra-high frequency ultrasounds, or micro-ultrasound, to bridge the gap for preclinical research needs. VisualSonics Vevo F2 ultrasound system operates at frequencies ranging from 1 MHz up to 71 MHz, which achieves spatial resolutions down to 30 microns.
Some applications and examples of vascular imaging measurements are:
• Assess anatomical and physical changes in vessels
• Intima-media thickness
• Atherosclerotic plaque identification
• Resistive Index
• Pulsatility Index
• Pulse wave velocity (PWV) to assess arterial stiffness.
In addition to ultrasound, VisualSonics offers a hybrid imaging system called Vevo F2 LAZR-X, which is a photoacoustic imaging platform. Photoacoustics merges optical imaging, using laser light, and ultrasound. The laser operates in the wavelength ranges of 680-970nm and 1200nm-2000nm. With these wavelengths it is possible to assess tissue oxygenation and image various contrast agents - allowing true molecular imaging capabilities for vascular researchers.
Micro-Ultrasound Applications
The field of vascular biology is broad, as the vascular system plays a role in the development and progression of many diseases and pathologies. Vascular research aims to uncover the underlying mechanisms of vessels as they relate to organ-specific diseases. Research in this area can be focused on angiogenesis, microcirculation/perfusion, inflammation, etc. as it relates to cancer, diabetes, atherosclerosis, stroke, and other pathologies.
Many diseases are associated with abnormalities in vessel walls and hemodynamic changes. To accurately assess these changes ultrasound features a variety of imaging modes including B-mode, M-mode, color Doppler, power Doppler, and pulsed-wave Doppler.
a. Anatomical Information
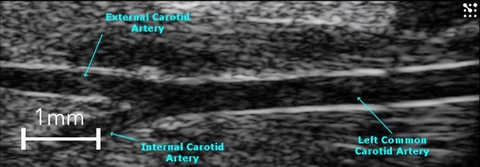
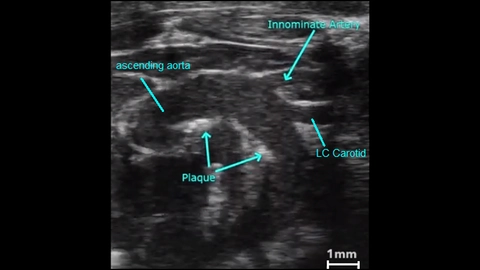
B-mode is the basic grey scale mode used to visualize the anatomy. Many vessels, including the aorta, pulmonary, carotid, etc. can be easily imaged in B-mode (Figure 1). This mode can be used to follow vessel remodeling or plaque formation over time [3].
M-Mode imaging, or “Motion-Mode” can be used to analyze vessel wall thickness as well as the movement of vessel walls over time. This can provide valuable information on the elasticity of the vessel wall as it changes in diameter from systole to diastole (Figure 2). Studies have shown that using both imaging modes described above are useful in evaluating carotid artery wall motion and plaque burden [4, 5]. Intima-media thickness of various vessels can also be assessed which is associated with cardiovascular risks [6-8] and can be used as a predictor of major cardiac events [9].
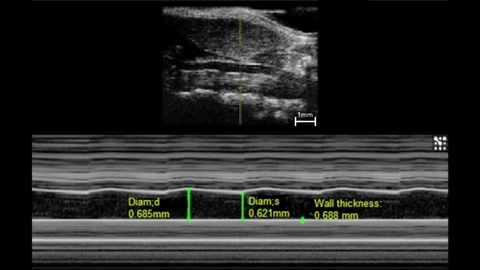
Measurements: wall thickness and vessel diameter in systole and diastole.
b. Hemodynamics
Further interrogation of vascular changes in preclinical animal models can be achieved by visualizing and quantifying hemodynamic changes with the use of color Doppler, power Doppler, and pulse wave (PW) Doppler. Color and Power Doppler enable visualization of either directional or non-directional blood flow, respectively. The ability to observe blood flow in real-time is helpful in the evaluation of valvular disease, including stenosis and regurgitation [10, 11].
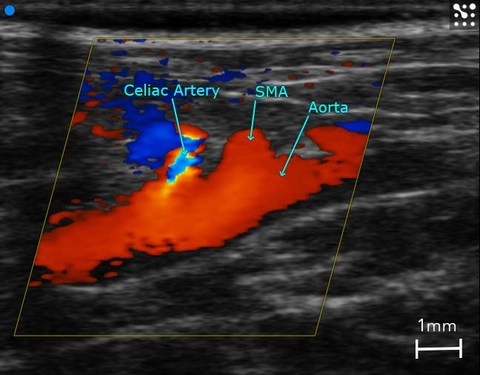
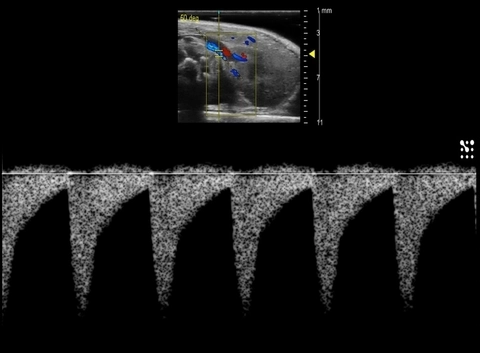
Color Doppler is also a critical tool to guide where to place sample volumes for PW Doppler which is used to display blood flow velocity (Figures 3 & 4). Changes in blood flow velocity, and in particular sheer stress [12], resistive index, and pulsatility index are calculated using PW Doppler measurements. Changes in these parameters can be prognostic indicators in abdominal aortic aneurysm [13], kidney disease [14], diabetic nephropathy [15], or placental insufficiency [16].
| 
|
c. 3D and 4D Imaging
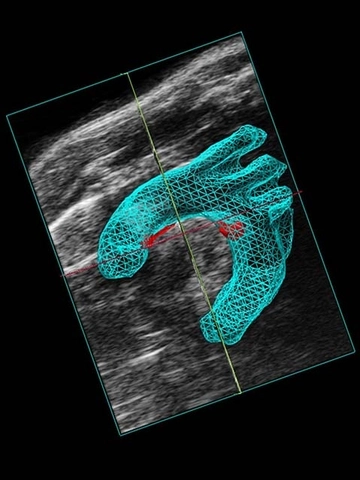
3D imaging provides accurate volume quantification. For example, if a plaque is identified in a vessel using 2D ultrasound, 3D imaging can be used to generate images for plaque volume characterization (Figure 5a, 5b). 3D imaging has been reliably used for abdominal aorta segmentation and volume analysis [13].
| 
|
4D imaging is one of the newest developments by VisualSonics that is a blend of 3D imaging plus high temporal resolution using EKV acquisition. EKV (or ECG gated Kilohertz Visualization) data is taken at every time point along the cardiac cycle during 3D image acquisition (Figure 6), allowing one to see the dynamics of a blood vessel. 4D removes assumptions regarding shape or dynamics of vessels including volumetric changes, area changes, and linear measurements on individual frames.
|
| Figure 6: 4D image of a mouse abdominal aorta. |
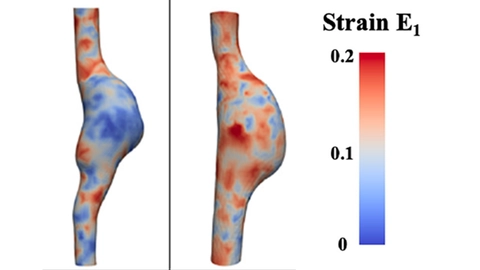
4D imaging has enhanced researchers’ ability to understand the complex dynamics associated with vessel kinetics and geometry. Cebull et al. have developed a novel, customized image acquisition and processing method that allows strain mapping of 4D ultrasound in abdominal aortic aneurysms (Figure 7) [17].
d. Contrast Enhanced Ultrasound (CEUS)
Ultrasound contrast agents are used to assess macrovascular and microvascular perfusion. Gas filled bubbles which have been optimized for high frequency ultrasound can be intravenously injected and easily detected within a specific region of interest. The microbubbles are restricted to the vascular space and true tissue perfusion (down to the capillary level) can be quantified based on the kinetics of the contrast microbubbles. This technique has been adopted in vascular medicine to help evaluate extracardiac events of ischemia and neovascularization in diseases such as atherosclerosis [18], and peripheral artery disease [19-20].
Microbubbles can also be targeted to vascular ligands of importance using streptavidin and biotin coupling, enabling ultrasound molecular imaging. The ability to detect and monitor differential expression of molecules within the vasculature can be used as a prognostic indicator and aid in the diagnosis of disease. Targeted microbubbles are particularly of interest in atherosclerotic plaque development because angiogenic events are unable to be detected with current diagnostic imaging modalities [21]. Over the past several years ultrasound molecular imaging has gained traction. Some promising targeted contrast agents demonstrate high potential for clinical translatability [22] or have received FDA approval (BR55, VEGFR2 target by Bracco).
e. Advanced Vascular Analysis
In addition to conventional approaches used to derive vascular parameters (described above), semi-automated approaches have been developed that provide a more in depth understanding of vascular disease and remodeling. Vevo Vasc is an analysis package that applies advanced speckle tracking algorithms to quantify vessel wall displacement during a cardiac cycle (Figure 8).
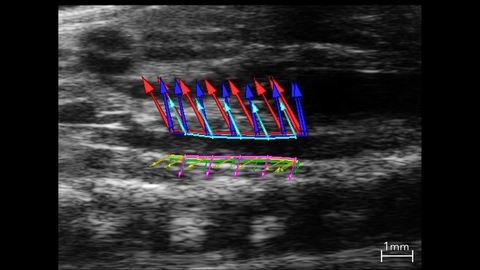
Strain, which is a measurement of this displacement, is a useful indicator of a vessels mechanical properties, such as stiffness. In atherosclerosis of the coronary artery [23], plaque development leads to vessel stiffening and is associated with a reduction in strain. Similarly, strain is reduced in intimal hyperplasia [24], where stiffness increases due to thickening of the tunica intima.
Pulse propagation velocity is another advanced tool that measures flow velocity by taking the ratio between the change in blood flow and the change in cross-sectional area of the vessel following a cardiac cycle [25]. With Vevo Vasc it is possible to estimate these changes across the entire length of the vessel. Similar to strain, these measurements can provide a good indication of vessel stiffness and could be useful to detect subtle changes in vascular diseases [26].
Photoacoustic Imaging Applications
Photoacoustic imaging is an emerging biomedical imaging technique that combines the high specificity of optical imaging techniques with the spatial resolution of ultrasound [27]. Using photoacoustic imaging, it is possible to measure several endogenous molecules including hemoglobin, melanin, collagen, and lipids. There are also a variety of dyes and nanoparticles that can administered in order to enhance photoacoustic imaging contrast. The Vevo LAZR-X system enables imaging of both endogenous and exogenous absorbers.
a. Hemoglobin
Hemoglobin is an abundant molecule present in red blood cells that provides strong photoacoustic contrast within the near infrared light range. The binding of oxygen to hemoglobin modifies its optical properties, making it possible to distinguish differences in oxy- and deoxyhemoglobin content. The Vevo LAZR-X Oxy-Hemo mode takes advantage of these differences to provide information on both tissue hemoglobin levels as well as blood oxygen saturation.
Photoacoustic imaging creates opportunities to study vascular biology through mapping blood vessel structure, assessing tissue perfusion, and detecting hypoxia [28]. The safety and feasibility of photoacoustic imaging for visualizing normal vasculature has been demonstrated in healthy human subjects [29, 30]. Likewise, photoacoustic imaging is capable of detecting vascular abnormalities in subjects with arteriovenous malformations [31] or within tumor tissues [32].
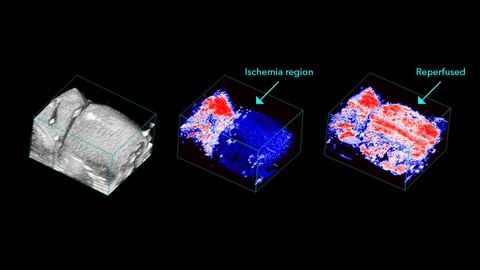
In cases of vascular injury, photoacoustic imaging allows for determination of thrombi location and size, and can help measure changes in tissue perfusion following occlusion [33]. Photoacoustic based hemoglobin measurements have also been performed in mouse models of peripheral arterial disease [34]. These studies reveal reduced tissue perfusion that are accompanied by increased deoxyhemoglobin levels. These measurements are also useful when evaluating novel therapeutics to restore vessel function after injury [35]. Similar observation have also been made in patients with Reynaud’s disease [36].
The ability of photoacoustic imaging to measure blood oxygenation levels has garnered considerable interest in the research community. Reduction in blood oxygenation levels are a good indicator of vascular injury and vessel occlusion [34], as loss of perfusion leads to increased hypoxia (Figure 9). Photoacoustic oxygen saturation measurements can also be performed in the brains of small animals [37] to identify regions of ischemic stroke [38]. The oxygenation status of chronic, hard-to-heal wounds is an area of increasing interest for wound therapy. Photoacoustic imaging can provide a non-invasive approach to assess wound oxygenation levels and evaluate new treatments that improve oxygenation [39].
b. Lipids
Photoacoustic imaging is capable of detecting endogenous signals from lipids within tissues. These signals are typically measured in the second near infrared window, between 1200-2000 nm. Given the lipid rich nature of plaques in atherosclerosis [40], photoacoustic imaging may provide clinicians with critical information on the risk of plaque rupture and help guide treatment.
Studies in ex vivo carotid [41] and aorta [42] samples highlight the ability of photoacoustic imaging to measure plaque lipid content, and reveal good spatial agreement with histology. Longitudinal photoacoustic imaging studies in mouse models of atherosclerosis also show the potential to detect plaque development over time [43].
Researchers are now examining miniaturized photoacoustic imaging probes that can be used for intravascular photoacoustic imaging [44-45]. Importantly, these approaches provides a non-destructive method to measure lipid content.
c. Collagen
Collagen is an endogenous absorber present in the connective tissues that line vessels to provide additional strength and maintain elasticity. Although collagen is not a dominate absorber in the near infrared region, it can be measured through multi-wavelength photoacoustic imaging and the advanced spectral unmixing tool available in Vevo LAB. Photoacoustic imaging of vessel collagen levels has not been performed to date, however it has been utilized to measure increased collagen deposition in kidney fibrosis models [46].
As atherosclerotic plaques are known to develop a fibrous collagenous cap, this could be another method to examine their underlying biology. Photoacoustic imaging of collagen may also be useful in studying other vascular diseases that involve fibrosis and vessel narrowing.
d. Contrast Agents
A variety of exogenous agents have been developed to enhance photoacoustic imaging contrast. Untargeted contrast agents provide useful information on tissue perfusion and vascular permeability. Optical dyes such as methylene blue, indocyanine green (ICG), and evans blue are common untargeted agents. In ischemic stroke models, injection of evans blue can be applied to study contrast leakage in the infarcted area [47], while in tumor models, ICG is used frequently to assess tumor perfusion and vessel permeability [48].
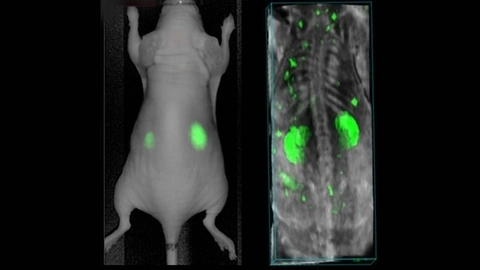
Targeted agents provide an additional level of specificity to contrast imaging. Near infrared erythrocyte-derived transducers (NETs) have been developed to accumulate in sites of stenosis or blockage in coronary artery disease [49]. AngiostampTM800 is a commercially available agent that targets αvβ3 integrin and provides excellent photoacoustic contrast [50]. The αvβ3 integrin is overexpressed on the surface of activated endothelial cells and therefore is a marker of angiogenesis and neovascularization (Figure 10).
Another strategy is to place the contrast agent into a living cell that can migrate through the body. For instance, loading macrophages with gold nanoparticles can be exploited to measure macrophage migration into atherosclerotic plaques [51]. Although these are a few examples, there is tremendous potential for contrast enhanced photoacoustic imaging in vascular disease.
e. PA EKV
Photoacoustic EKV (PA EKV) is an advanced photoacoustic acquisition mode available on the Vevo LAZR-X that is built on similar principles as ultrasound EKV. In PA EKV, photoacoustic images are acquired over several cardiac cycles and averaged to create a signal dataset with high temporal resolution.
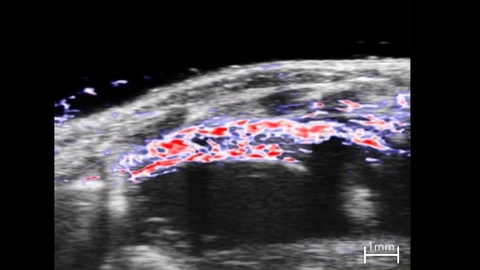
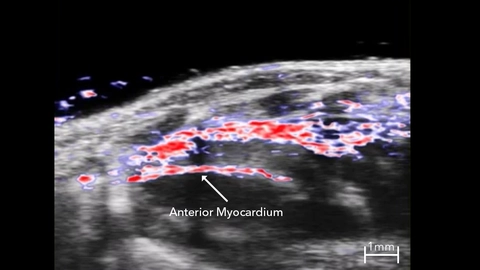
In Oxy-Hemo mode, PA EKV can measure hemoglobin and oxygen saturation levels in the myocardium throughout the cardiac cycle (Figure 11). PA EKV can be useful for studying cardiovascular disease, including myocardial infarction, where MI results in reduced oxygen saturation levels in the anterior myocardium that recover slowly over several days as neoangiogenesis is stimulated [52]. Oxy-Hemo PA EKV can also be applied for vascular imaging to assess changes in vessel hemodynamics.
Using a multi-spectral acquisition mode, PA EKV can be combined with exogenous contrast agents to perform cell tracking and targeted molecular imaging for cardiac and vascular applications.
| 
|
Conclusions and Future Outlook
High frequency ultrasound and photoacoustic imaging represent a diverse toolbox to diagnosis vascular disease and study underlying disease biology. While ultrasound provides insight into changes in vessel structure and hemodynamics, photoacoustic imaging creates opportunities to assess hypoxia and characterize molecular changes that occur throughout the progression of disease.
Advanced acquisition methods such as 4D imaging and EKV enables acquisition of high frame rate datasets that can be combined with state-of-the-art analysis algorithms to detect subtle mechanical changes in vessel function.
With the release of the new Vevo F2 imaging platform, VisualSonics gives researchers the power to develop the next-generation of ultrasound and photoacoustic imaging tools. This is accomplished using the F2 system Vevo Advanced Data Acquisition (VADA) mode, which allows researchers to access and customize the ultrasound signal transmit and receive channels.
Super-resolution imaging is one field that seeks to overcome the inherent resolution limits of conventional ultrasound by detecting single microbubbles in circulation. This approach can provide detailed vascular network images and is capable of visualizing small abnormalities in the vessel wall in atherosclerosis [53].
Plane wave imaging is another field of research which is pushing the limits of ultrasound imaging speeds, with frame rates exceeding 1000 frames per second [54]. Furthermore, shear wave elastography is an advanced technique which is able to directly measure tissue stiffness and can be applied in a variety of pathologies.
Combining these advanced techniques with high frequency ultrasound and photoacoustic imaging will allow researchers to study vascular disease and biology in a capacity never before possible.
References
1. Foster FS, Zhang MY, Zhou YQ, Liu G, Mehi J, Cherin E, Harasiewicz KA, Starkoski BG, Zan L, Knapik DA, Adamson SL. A new ultrasound instrument for in
vivo microimaging of mice. Ultrasound in medicine & biology. 2002 Sep 1;28(9):1165-72
2. Lockwood GR, Turnball DH, Christopher DA, Foster FS. Beyond 30 MHz [applications of high-frequency ultrasound imaging]. IEEE Engineering in Medicine and Biology Magazine. 1996 Nov;15(6):60-71.
3. Harmon EY, Fronhofer V, Keller RS, Feustel PJ, Brosnan MJ, et al. Ultrasound Biomicroscopy for Longitudinal Studies of Carotid Plaque Development in Mice: Validation with Histological Endpoints. PLoS ONE 2012;7(1): e29944. doi:10.1371/journal. pone.0029944
4. Svedlund S, Gan LM. Longitudinal common carotid
artery wall motion is associated with plaque burden in man and mouse. Atherosclerosis. 2011 Jul;217(1):120- 4. doi: 10.1016/j.atherosclerosis.2011.02.046. Epub 2011 Mar 3.
5. Ni M, Zhang M, Ding SF, Chen WQ, Zhang Y. Micro-ultrasound imaging assessment of carotid plaque characteristics in apolipoprotein-E knockout mice. Atherosclerosis. 2008 Mar;197(1):64-71. doi: 10.1016/j.atherosclerosis.2007.07.039. Epub 2007 Sep 17.
6. Sarkola T, Slorach C, Hui W, Bradley TJ, Redington AN, Jaeggi E. Transcutaneous very-high resolution ultrasound for the quantification of carotid arterial intima-media thickness in children - feasibility and comparison with conventional high resolution vascular ultrasound imaging. Atherosclerosis. 2012 Sep;224(1):102-7. doi: 10.1016/j. atherosclerosis.2012.06.054. Epub 2012 Jun 27.
7. Eklund C, Friberg P, Gan LM. High-resolution radial artery intima-media thickness and cardiovascular risk factors in patients with suspected coronary artery disease--comparison with common carotid artery intima-media thickness. Atherosclerosis. 2012 Mar;221(1):118-23. doi:10.1016/j. atherosclerosis.2011.12.035. Epub 2011 Dec 29.
8. Razuvaev A, Lund K, Roy J, Hedin U, Caidahl K. Noninvasive real-time imaging of intima thickness after rat carotid artery balloon injury using ultrasound biomicroscopy. Atherosclerosis. 2008 Aug;199(2):310- 6. doi: 10.1016/j.atherosclerosis.2007.11.035. Epub 2008 Jan 10.
9. Eklund C, Omerovic E, Haraldsson I, Friberg P, Gan LM. Radial artery intima-media thickness predicts major cardiovascular events in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014 Jul;15(7):769-75. doi: 10.1093/ehjci/ jet285. Epub 2014 Jan 26.
10. Casaclang-Verzosa G, Enriquez-Sarano M, Villaraga, HR, Miller, JD. Echocardiographic approaches and protocols for comprehensive phenotypic characterization of valvular heart disease in mice. J. Vis. Exp. 2017 (120), e54110 .doi:10.3791/54110 (2017).
11. Thomas JD. Doppler echocardiographic assessment of valvar regurgitation. Heart 2002; 88:651-657. 12. Swanson TA, Conte T, Deeley B, Portugal S, Kreeger JM, Obert LA, Joseph EC, Wisialowski TA, Sokolowski SA, Rief C, Nugent P, Lawton MP, Enerson BE. Hemodynamic correlates of drug-induced vascular injury in the rat using high-frequency ultrasound imaging. Toxicol Pathol. 2014 Jun;42(4):784-91. doi: 10.1177/0192623314525687. Epub 2014 Mar 26.
13. Sangha GS, Busch A, Acuna A, Berman AG, Phillips EH, Trenner M, Eckstein HH, Maegdefessel L, Goergen CJ. Effects of Iliac Stenosis on Abdominal Aortic Aneurysm Formation in Mice and Humans. J Vasc Res. 2019; 56(5):217-229. doi: 10.1159/000501312. Epub 2019 Jul 4.
14. Radermacher J, Ellis S, Haller H: Renal resistance index and progression of renal disease. Hypertension 2002;39:699-703.
15. Xu H, Ma Z, Lu S, Li R, Lyu L, Ding L, Lu Q. Renal resistive index as a novel Indicator for renal complications in high-fat diet-fed mice. Kidney Blood Press Res 2017;42:1128-1140. doi:10.1159/000485781.
16. Hernandez-Andrade, Ahn H, Szalai G, Korzeniewski SJ, Wang B, King M, Chaiworapongsa T, Than NG, Romero R. Evaluation of utero-placental and fetal hemodyanamic parameters throughout gestation in pregnant mice using high-frequency ultrasound. Ultrasound Med Biol. 2014; 40(2): 351–360. doi:10.1016/j.ultrasmedbio.2013.09.026.
17. Cebull HL, Soepriatna AH, Boyle JJ, Rothenberger SM, Goergen CJ. Strain mapping from fourdimensional ultrasound reveals complex remodeling in dissecting murine abdominal aortic aneurysms. Journal of Biomed Engineering 2019;141. doi: 10.1115/1.4043075.
18. Mehta KS, Lee JJ, Taha AA, Avgerinos E, Chaer RA. Vascular applications of contrast-enhanced ultrasound imaging. J Vasc Surg 2017;66:266-74. doi:10.1016/j. jvs2016.12.133.
19. Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, Kaul S, Patrie JT. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging 2008;1:343–350. doi:10.1016/j.jcmg.2008.04.001.
20. Baltgalvis KA, White K, Li W, Claypool MD, Lang W, Alcantara R, Singh BK, Friera AM, McLaughlin J, Hansen D, McCaughey K, Nguyen H, Smith IJ, Godinez G, Shaw SJ, Goff D, Singh R, Markovtsov V, Sun TQ, Jenkins Y, Uy G, Li Y, Pan A, Gururaja T, Lau D, Park D, Hitoshi Y, Payan DG, Kinsella TM. Exercise performance and peripheral vascular insufficiency imprve with AMPK activation in high-fat diet-fed mice. Am J Physiol Heart Circ Physiol. 2014;306(8):H1128- 45. doi:10.1152/ajpheart.00839.2013.
21. Daeichin V, Kooiman K, Skachkov I, Bosch JG, Theelen TL, Steiger K, Needles A, Janssen BJ, Daemen MJ, van der Steen AF, de Jong N, Sluimer JC. Quantification of Endothelial αvβ3 Expression with High-Frequency Ultrasound and Targeted Microbubbles: In Vitro and In Vivo Studies. Ultrasound Med Biol. 2016 Sep;42(9):2283-93. doi: 10.1016/j. ultrasmedbio.2016.05.005. Epub 2016 Jun 11.
22. Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: moving towards clinical translation. Eur J Radiol. 2015 Sep;84(9):1685-1693. Doi:10.1016/j.erad.2015.03.016.
23. Svedlund S, Gan LM. Longitudinal common carotid artery wall motion is associated with plaque burden in man and mouse. Atherosclerosis. 2011 Jul 1;217(1):120-4.
24. Favreau JT, Liu C, Yu P, Tao M, Mauro C, Gaudette GR, Ozaki CK. Acute reductions in mechanical wall strain precede the formation of intimal hyperplasia in a murine model of arterial occlusive disease. Journal of vascular surgery. 2014 Nov 1;60(5):1340-7.
25. Williams R, Needles A, Cherin E, Zhou YQ, Henkelman RM, Adamson SL, Foster FS. Noninvasive ultrasonic measurement of regional and local pulsewave velocity in mice. Ultrasound in medicine & biology. 2007 Sep 1;33(9):1368-75.
26. Sharma N, Sun Z, Hill MA, Hans CP. Measurement of pulse propagation velocity, distensibility and strain in an abdominal aortic aneurysm mouse model. JoVE (Journal of Visualized Experiments). 2020 Feb 23(156):e60515.
27. Needles A, Heinmiller A, Sun J, Theodoropoulos C, Bates D, Hirson D, Yin M, Foster FS. Development and initial application of a fully integrated photoacoustic micro-ultrasound system. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2013 May 3;60(5):888-97.
28. Karlas A, Fasoula NA, Paul-Yuan K, Reber J, Kallmayer M, Bozhko D, Seeger M, Eckstein HH, Wildgruber M, Ntziachristos V. Cardiovascular optoacoustics: From mice to men–A review. Photoacoustics. 2019 Jun 1;14:19-30.
29. Matsumoto Y, Asao Y, Yoshikawa A, Sekiguchi H, Takada M, Furu M, Saito S, Kataoka M, Abe H, Yagi T, Togashi K. Label-free photoacoustic imaging of human palmar vessels: a structural morphological analysis. Scientific reports. 2018 Jan 15;8(1):1-8.
30. Sheikh R, Hammar B, Naumovska M, Dahlstrand U, Gesslein B, Erlöv T, Cinthio M, Malmsjö M. Photoacoustic imaging for non-invasive examination of the healthy temporal artery–systematic evaluation of visual function in healthy subjects. Acta ophthalmologica. 2020 Aug 25.
31. Masthoff M, Helfen A, Claussen J, Karlas A, Markwardt NA, Ntziachristos V, Eisenblätter M, Wildgruber M. Use of multispectral optoacoustic tomography to diagnose vascular malformations. JAMA dermatology. 2018 Dec 1;154(12):1457-62.
32. Pourebrahimi B, Al-Mahrouki A, Zalev J, Nofiele J, Czarnota GJ, Kolios MC. Classifying normal and abnormal vascular tissues using photoacoustic signals. Proc. SPIE 8581, Photons Plus Ultrasound: Imaging and Sensing 2013, 858141.
33. Li B, Fu C, Ma G, Fan Q, Yao Y. Photoacoustic imaging: a novel tool for detecting carotid artery thrombosis in mice. Journal of Vascular Research. 2017;54(4):217-25.
34. Hedhli J, Kim M, Knox HJ, Cole JA, Huynh T, Schuelke M, Dobrucki IT, Kalinowski L, Chan J, Sinusas AJ, Insana MF. Imaging the landmarks of vascular recovery. Theranostics. 2020;10(4):1733.
35. Das D, Pramanik M. Combined ultrasound and photoacoustic imaging of blood clot during microbubble-assisted sonothrombolysis. Journal of biomedical optics. 2019 Jul;24(12):121902.
36. Eisenbrey JR, Stanczak M, Forsberg F, Mendoza- Ballesteros FA, Lyshchik A. Photoacoustic oxygenation quantification in patients with Raynaud’s: first-inhuman results. Ultrasound in Medicine & Biology. 2018 Oct 1;44(10):2081-8.
37. Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature biotechnology. 2003 Jul;21(7):803-6.
38. Hu S, Gonzales E, Soetikno B, Gong E, Yan P, Maslov K, Lee JM, Wang LV. Optical-resolution photoacoustic microscopy of ischemic stroke. InPhotons Plus Ultrasound: Imaging and Sensing 2011 2011 Feb 10 (Vol. 7899, p. 789906). International Society for Optics and Photonics.
39. Petri M, Stoffels I, Jose J, Leyh J, Schulz A, Dissemond J, Schadendorf D, Klode J. Photoacoustic imaging of real-time oxygen changes in chronic leg ulcers after topical application of a haemoglobin spray: a pilot study. Journal of wound care. 2016 Feb 2;25(2):87-91.
40. Gofman JW, Lindgren F, Elliott H, Mantz W, Hewitt J, Strisower B, Herring V, Lyon TP. The role of lipids and lipoproteins in atherosclerosis. Science. 1950 Feb 17;111(2877):166-86.
41. Kruizinga P, van der Steen AF, de Jong N, Springeling G, Robertus JL, van der Lugt A, van Soest G. Photoacoustic imaging of carotid artery atherosclerosis. Journal of biomedical optics. 2014 Nov;19(11):110504.
42. Wang B, Karpiouk A, Yeager D, Amirian J, Litovsky S, Smalling R, Emelianov S. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood. Optics letters. 2012 Apr 1;37(7):1244-6.
43. Sangha GS, Goergen CJ. Label-free photoacoustic and ultrasound imaging for murine atherosclerosis characterization. APL bioengineering. 2020 Jun 1;4(2):026102.
44. Zhang J, Yang S, Ji X, Zhou Q, Xing D.< Characterization of lipid-rich aortic plaques by intravascular photoacoustic tomography: ex vivo and in vivo validation in a rabbit atherosclerosis model with histologic correlation. Journal of the American College of Cardiology. 2014 Jul 29;64(4):385-90.
45. Jansen K, Van Der Steen AF, van Beusekom HM, Oosterhuis JW, van Soest G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Optics letters. 2011 Mar 1;36(5): 597-9.
46. Hysi E, He X, Fadhel MN, Zhang T, Krizova A, Ordon M, Farcas M, Pace KT, Mintsopoulos V, Lee WL, Kolios MC. Photoacoustic imaging of kidney fibrosis for assessing pretransplant organ quality. JCI insight 2020 May 21;5(10).
47. Lv J, Li S, Zhang J, Duan F, Wu Z, Chen R, Chen M, Huang S, Ma H, Nie L. In vivo photoacoustic imaging dynamically monitors the structural and functional changes of ischemic stroke at a very early stage. Theranostics. 2020;10(2):816.
48. Okumura K, Yoshida K, Yoshioka K, Aki S, Yoneda N, Inoue D, Kitao A, Ogi T, Kozaka K, Minami T, Koda W. Photoacoustic imaging of tumour vascular permeability with indocyanine green in a mouse model. European radiology experimental. 2018 Dec;2(1):5.
49. Liu Y, Hanley T, Chen H, Long SR, Gambhir SS, Cheng Z, Wu JC, El Fakhri G, Anvari B, Zaman RT. Non-Invasive Photoacoustic Imaging of In Vivo Mice with Erythrocyte Derived Optical Nanoparticles to Detect CAD/MI. Scientific reports. 2020 Apr 6;10(1): 1-9.
50. Lavaud J, Henry M, Gayet P, Fertin A, Vollaire J, Usson Y, Coll JL, Josserand V. Noninvasive monitoring of liver metastasis development via combined multispectral photoacoustic imaging and fluorescence diffuse optical tomography. International Journal of Biological Sciences. 2020;16(9):1616.
51. Wang, B., Yantsen, E., Larson, T., Karpiouk, A.B., Sethuraman, S., Su, J.L., Sokolov, K. and Emelianov, S.Y., 2009. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Letters, 9(6), pp.2212-2217.
52. Hackfort BT, Chalise U, Daseke MJ, Lindsey ML. Myocardial Oxygen Saturation Measured by Photoacoustic EKV Imaging. The FASEB Journal. 2020 Apr 1;34(S1).
53. Yu J, Lavery L, Kim K. Super-resolution ultrasound imaging method for microvasculature in vivo with a high temporal accuracy. Scientific reports. 2018 Sep 17;8(1).
54. Montaldo G, Tanter M, Bercoff J, Benech N, Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2009 Apr 21;56(3):489-506.