Submitted by Dr. Paola Rosas, Postdoctoral Research Associate at Dr. Carl Tong laboratory, Department of Medical Physiology, Texas A&M University Health Science Center.
We used the Vevo technology to periodically assess left ventricular function during aging and to determine the stage of worst disease condition, at which point we harvested the heart and performed papillary muscle studies. Functional changes found with the Vevo, matched our force generation and relaxation rate measurements obtained in intact papillary muscles.
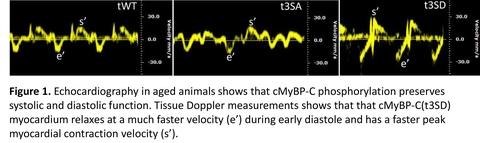
The purpose of this study is to determine the role of phosphorylated cardiac myosin binding protein-C (cMyBP-C) in supporting normal heart function during aging. cMyBP-C, a heart muscle thick filament protein, regulates cross-bridge attach/detachment process by its phosphorylation status; and we found that its phosphorylation status decreases with aging. For this study we generated phosphorylation-deficient cMyBP-C(t3SA):S273A/S282A/S302A, phosphorylation-mimetic cMyBP-C (t3SD):S273D/S282D/S302D and wild type cMyBP-C(tWT) mice models and aged them to 18 months in order to mimic 70-80 year old humans. Using echocardiography, we observed that phosphorylation-mimetic cMyBP-C(t3SD) hearts maintained an EF>45% throughout aging and exhibited enhanced systolic and diastolic function despite aging. Meanwhile, cMyBP-C(tWT) and non-phosphorylation deficient cMyBP-C(t3SA) hearts showed decline of systolic and diastolic function with aging. Moreover, cMyBP-C(t3SD) mice showed enhanced survival at 18 months. These results suggest that phosphorylation of cMyBP-C has a potential benefit and can be translated into novel treatments for age related cardiac dysfunction.
Vevo technology provides an excellent tool toward a comprehensive assessment of structural and functional changes that occur during aging; as well as it offers a great potential to help researchers that require a high throughput non-invasive technology for rapid data collection. Our next step will be to treat young and aged wild type mice with adenovirus containing phosphorylation-mimetic cMyBP-C plasmids and investigate whether diastolic dysfunction due to aging could be prevented or treated. For this approach Vevo technology will be an indispensable tool to assess and to determine the effectiveness of the treatment.
Dr. Paola Rosas presented her work at the American Heart Association Scientific Sessions in New Orleans, 2016. Dr. Rosas’ work is supported by a Postdoctoral Fellowship form the American Heart Association and she is a Postdoctoral Research Associate at Dr. Carl Tong laboratory, Department of Medical Physiology, Texas A&M University Health Science Center.