Submitted by Dr. Richard Bouchard, Assistant Professor, Department of Imaging Physics, The University of Texas MD Anderson Cancer Center
Hypoxia, a deficiency in the amount of oxygen reaching tissue, plays a central role in poor HCC outcomes. Accumulating evidence shows that up to 50-60% of locally advanced solid tumors may exhibit heterogeneously distributed hypoxic (and anoxic) areas within the tumor mass. Because the supply and consumption of oxygen fluctuate temporally as well as spatially, these heterogeneous hypoxia regions also vary with time. It has been shown that the level of hypoxia has predictive correlation with HCC response to sorafenib, which is the only drug that is clinically approved for patients with advanced HCC. Development of a robust diagnostic platform capable of imaging these heterogeneous regions can be used to further our understanding and quantify these different hypoxia subtypes while evaluating spatial and temporal heterogeneity, and thus potentially creating novel therapeutic opportunities.
Because photoacoustic (PA) imaging provides penetration depth well beyond a centimeter and sub-millimeter spatial resolution, it has the potential for a broader clinical translation than other forms of optical imaging, including depiction of transient oxygen saturation in orthotopic liver tumors. In our preliminary studies, baseline levels of oxygenation were assessed in a rat model of HCC using the Vevo 2100 LAZR system and the LZ201 array with a customized narrow irradiation output (~1 cm) over 2 minutes during inhalation of medical air. Thereafter, oxygen supply was increased to 100% O2 (2L/min). Oxygenation levels were registered live over 8 minutes, before changing back to medical air. The magnitude of signal increase observed before and after this ‘hyperoxia challenge’ (Figure 1. C-D) highlights the sensitivity of the technique. The oxygen saturation under pure oxygen supply is about 28% higher than under medical air supply.
Additionally, in order to fully characterize the relationship between tissue oxygenation and microvasculature, we have complemented our PAUS analysis with perfusion assessment through injection of non-targeted microbubbles (Figure 1. B and F). We acquired contrast-enhanced ultrasound imaging data immediately after an intravenous 150-microliter bolus injection of non-targeted microbubbles at a concentration of 2x109 microbubbles per ml. Tumor perfusion and peak enhancement at different ROIs were quantified with VevoCQ™ Software. 3D imaging data also was acquired before injection (baseline) and during the early portion of the wash-out phase via a 3D acquisition to provide a qualitative, volumetric assessment of perfusion throughout the tumor (Figure 1. E). The analysis shows about 10% percent of contrast enhancement within the tumor volume.
As we move past the pilot phase of this study and start imaging a larger, statically significant coherent, we are encouraged that the Vevo LAZR with micro-bubble perfusion will provide us with valuable insight into perfusion and oxygen saturation dynamics in the tumor microenvironment of an orthotopic rat model of HCC. This work was recently funded with a Cancer Prevention and Research Institute of Texas grant (#RP160229).
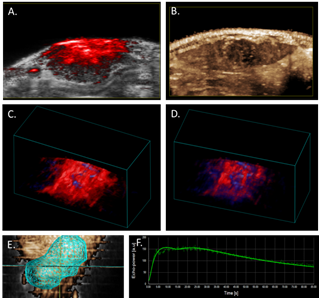
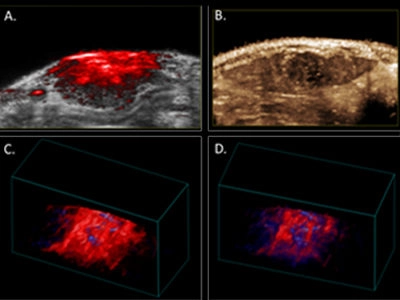
Figure 1. Photoacoustic and contrast-enhanced ultrasonic imaging in the HCC Buffalo rat orthotopic model.
A. Photoacoustic image of rat liver tumor overlaid B-mode image.
B. Contrast-enhanced perfusion, maximum intensity projection image, illustrating heterogeneous microbubble perfusion in the tumor.
C. PA 3D-image of oxy/deoxy-Hb heterogeneity throughout the tumor under 100% oxygen; and
D. under medical air (21%).
E. 3D tumor segmentation of contrast-enhanced ultrasonic image. F. Wash-in/wash-out curve of microbubble signal intensity in tumor over time follow bolus injection.